Rapid urease tests – such as the ULTRA-FAST UFT300 H. pylori Quick Test from BIOHIT HealthCare – are helping to quickly determine H. pylori infection during endoscopy procedures. However, these tests often operate outside the oversight of point-of-care testing (POCT) teams responsible for maintaining testing standards. In this blog, we explore the importance of bridging the gap between endoscopy and POCT teams, the role of internal quality controls in ensuring reliable results, and how H. pylori Control+ can enhance diagnostic confidence.
Why is H. pylori a concern?
The Gram-negative bacterium H. pylori is thought to colonise the stomach of around 30 per cent of people in the UK and, while the majority are asymptomatic, approximately 15 per cent encounter gastrointestinal problems. Chronic infection can trigger inflammation in the stomach lining, which can progress to atrophic gastritis, a condition strongly associated with peptic ulcer disease and increased risk of gastric cancer. In fact, H. pylori infection is estimated to be associated with 95 per cent of duodenal ulcers and 70-80 per cent of gastric ulcers,1 and the international agency for research on cancer (IARC) of the World Health Organisation has classified the microorganism as a group 1 carcinogen since 1994. The reliable, rapid detection of H. pylori is therefore extremely helpful to clinicians, guiding them to prescribe appropriate eradication treatment as soon as possible to prevent the onset of more severe and irreversible diseases.
The importance of rapid H. pylori detection
Detecting H. pylori infection is essential for diagnosing and managing upper gastrointestinal disorders. There are several methods currently in routine use for detecting H. pylori infection, including rapid urease tests, stool antigen tests and urease breath tests. For patients already referred for endoscopy, rapid urease tests provide a convenient way to assess biopsy samples during the procedure, helping clinicians to quickly pinpoint the cause of discomfort. This approach allows endoscopy teams to provide more targeted care, improving outcomes for patients with suspected infections or other gastric issues.
The UFT300 H. pylori Quick Test has revolutionised this process, offering fast and reliable results for busy endoscopy settings. Unlike conventional urease tests, the UFT300 delivers results in as little as five minutes, allowing clinicians to input findings directly into the endoscopy reporting system and discuss them with patients before they leave the procedure room. Over 275 hospitals in the UK alone have already adopted the UFT300 H. pylori Quick Test, with countless services worldwide benefiting from its speed and reliability. This test has set a new standard for diagnosing H. pylori infection during endoscopy appointments, streamlining workflows and enhancing patient care.
Who ensures the quality of urease tests?
Rapid urease tests such as the BIOHIT UFT300 H. pylori Quick Test are used by every endoscopy department in the UK. However, this testing is not always administrated by POCT teams, which play a critical role in ensuring that POCT systems are functioning correctly and yielding reliable patient results. This includes both training and implementation of internal quality control schemes, including systematic analysis of control materials – often supplied by the test manufacturer, as would be the case for laboratory-based tests – before processing patient samples. 2,3 The systematic recording of these internal quality control results is essential to demonstrate compliance with quality standards, as well as to maintain trust in diagnostic results for clinicians and patients alike.
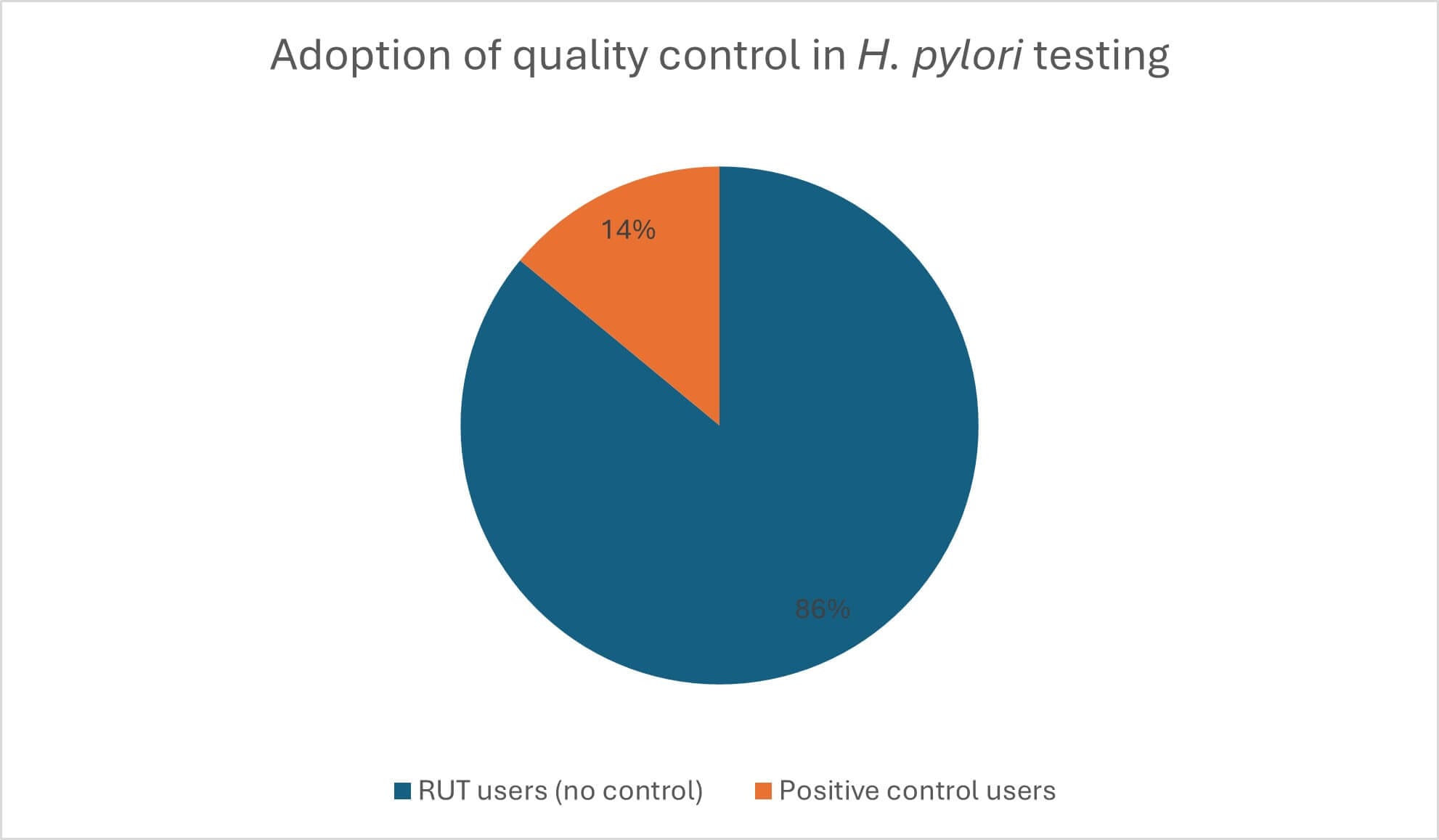
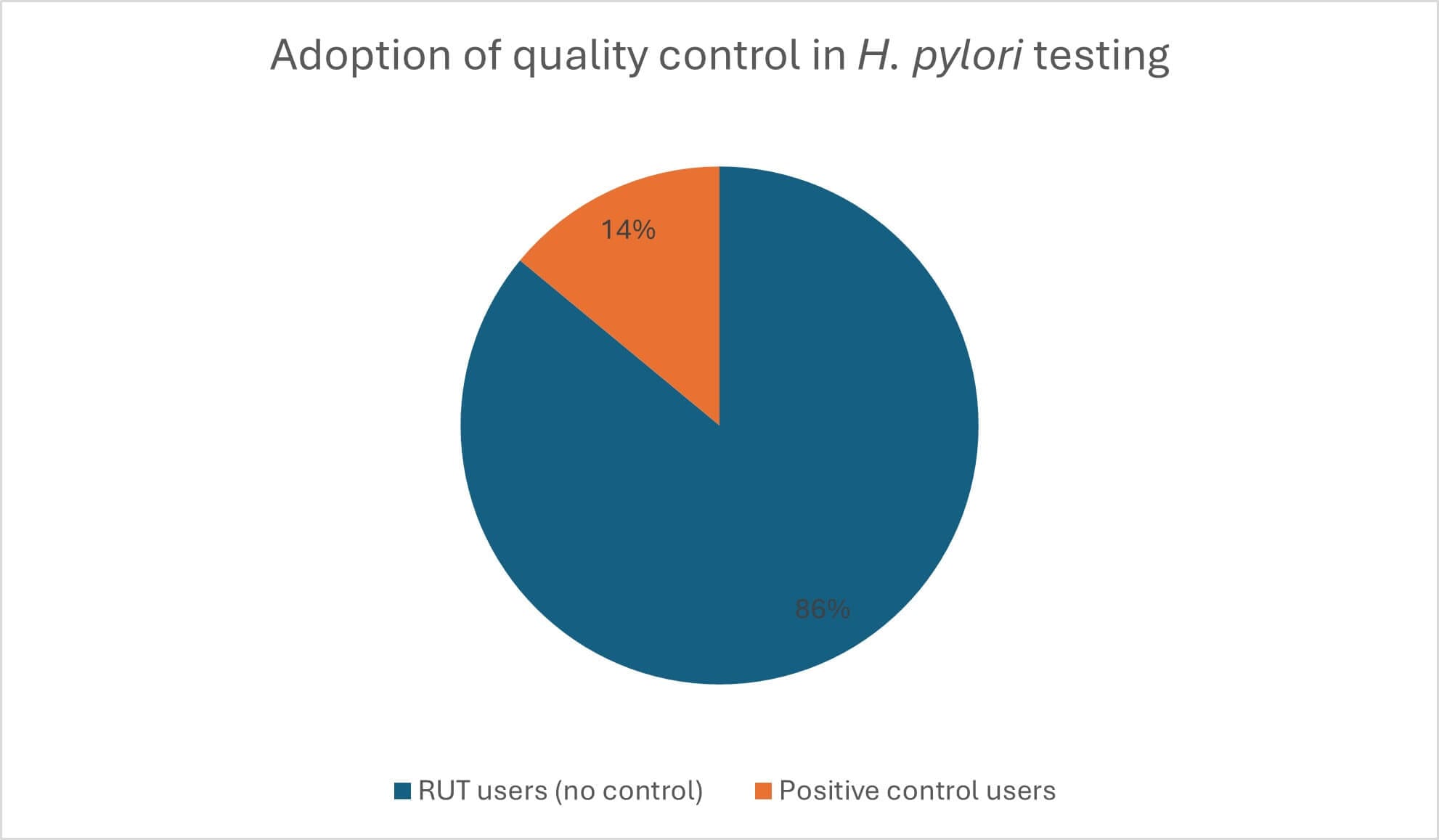
Many endoscopy teams may not realise the importance of POCT oversight, and analysis of the number of departments using UFT300 compared to those purchasing positive controls – such as H. pylori Control+ – has brought to light a disconnect between testing and quality assurance across the country (Figure 1). Raising awareness of this issue is vital to ensure that rapid urease tests are treated like any other diagnostics, and managed to the same high standards. By working collaboratively, POCT and endoscopy teams can strengthen quality assurance and optimise the performance of diagnostic tools, ensuring better outcomes for patients.
Figure 1: Only 14 per cent of ULTRA-FAST UFT300 H. pylori Quick Test users rely on H. pylori Control+ to verify test performance.
Confirming reliable urease test performance
BIOHIT offers a positive urease control – H. pylori Control+ – to help clinical teams verify the performance and consistency of H. pylori urease tests. This straightforward solution enables daily quality checks to confirm test performance before processing patient samples. Supplied in a convenient 1 ml dropper bottle, it provides sufficient material for up to 100 quality control tests. Integrating H. pylori Control+ into testing protocols helps endoscopy teams to demonstrate and maintain the reliability of rapid urease tests and uphold stringent quality assurance standards.
Find out more about the UFT300 H. pylori Quick Test and H. pylori Control+, or read how Northern Care Alliance is using these tests.